After using the iPad2 and the Apple TV to mirror iPad content through my LCD projector, I realized that this setup can be used as a wireless document camera by using the built-in camera app on the iPad2.
The Setup:
First of all, I setup a ‘stand’ for the iPad, so that it could project anything underneath it. Being a science teacher, I have access to plenty of lab stands and clamps (I actually wrapped the two metal rods in electrical tape to protect the iPad2 from scratches).
I gently rested the iPad2 on the stand, being careful to center the camera on the lab table below, and secured it with a large rubber-band.
I found that I needed a wide stand so that students could fit their whiteboards underneath without difficulty.
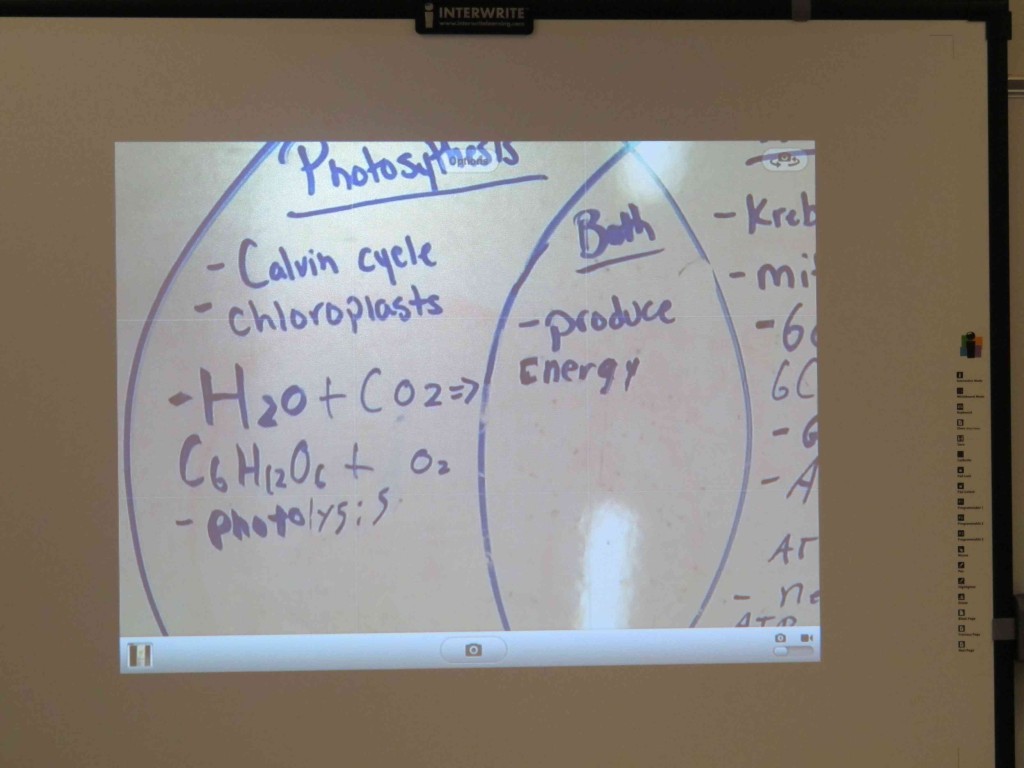
This system is also flexible, as it is wireless. I can carry it back to the lab and showcase individual student work to help direct a laboratory investigation. Taking a picture, I was even able to annotate over a photo by importing it through an app like the Educreactions Interactive Whiteboard app.















Recent Comments